Protocols
Workpackage 1
Materials
sterile 15 ml falcon tube
glass tubes
tube racks
DensiCHEK plus Biomerieux
Vortex
100-1000 mcl mechanical pipette
1 ml sterile insulin syringe
serum from healthy volunteers
saline sterile solution
MH broth
BacT/ALERT FA FAN® Aerobic Biomeriuex blood culture bottles
BacT/ALERT SA Standard Aerobic Biomerieux blood culture bottles (without inactivating matrix)
Bact/ALERT 3D Biomerieux incubator system
Test isolates
Bacterial strains were obtained from the banked collection of the Microbiology Unit, Hub Laboratory, AUSL Romagna, Cesena, Italy.
Each day before testing, strains are thawed and incubated at 37°C for 18-24 hours.
MICs were confirmed by Etest using the manufacturer’s recommendations
| Isolate | Abbreviation | Enzyme | Source | Ceftazidime/Avibactam MIC (mg/L) | Ceftazidime | Gentamicin | Meropenem | Tigecycline | Meropenem-Vaborbactam | Colistin | Ciprofloxacin | Ampicillin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Klebsiella pneumonia | KI_WT | Wild type | clinical isolate | 0.125 | 0.125 | 1 | <=0.06 | |||||
| Klebsiella pneumoniae ESBL ATCC 700603 | KP_ATCC | bla SHV-18 | Reference strain | 0.75 | 12 | 0.03 | 4 | |||||
| Klebsiella pneumoniae KPC- A | KPC_A | KPC-3 | Clinical isolate | 2 | 2 | 0.75 | 0.38 | |||||
| Klebsiella pneumoniae KPC- B | KPC_B | KPC-3 | Clinical isolate | 1 | 0.5 | 32 | 0.38 | |||||
| Klebsiella pneumoniae KPC- C | KPC_C | KPC-3 | Clinical isolate | >256 | ||||||||
| Klebsiella penumonia Catannia | KPC-Catania | Clinical isolate | 16 | |||||||||
| Klebsiella pneumoniae NDM | KP_NDM | NDM-2 | Clinical isolate | >256 | >256 | |||||||
| Klebsiella pneumonia VIM | KP_VIM | VIM | Clinical isolate | >256 | >256 | |||||||
| Escherichia coli ATCC 25922 | EC_ATCC | ATCC | 0.19 | 0.125 | 0.012 | 4 | ||||||
| Klebsiella pneumonia | KP_Dam | Clinical isolate | 4 | |||||||||
| Klebsiella pneumonia | KP_FAB | ESBL | ||||||||||
| Klebsiella pneumonia | KP_Cav | 16 | 4 | |||||||||
| Escherichia coli | EC_Mont | 64 | >32 | 4 | ||||||||
| Escherichia coli | EC_Rossi | 8 | >32 | |||||||||
| Pseudomonas aeruginosa ATCC 27853 | PA_ATCC | 1 | 0.38 | 0.125 | ||||||||
| Pseudononas aeruginosa | PA_Fabri | >32 |
MICs were performed and confirmed by Etest
Inoculum preparation
The proposed method are a modification of Tpos assay as proposed by Kaltsas et al.
Dilution schemes are from the Clinical Laboratory Standards Institute (CLSI) M21-A Methodology for the Serum Bactericidal Test and M26A Methodology for Determining Bactericidal Activity of Antibiotics.
To prepare a standardized inoculum (1.5 x102 CFU/mL) for injection in blood culture bottles:
Colonies are selected from an 18- to 24-hour agar plate and suspended in sterile 0.9% phosphate buffered saline (PBS) in a glass test tube. The suspension is then adjusted by optimal density using a Densichek turbidity meter to an equivalent to a 0.5 McFarland standard corresponding to 1 to 2 × 108 CFU/mL.
The inoculum is then serially diluted using ten-fold dilution scheme: 200 μL inoculum dispensed by micropipette into 1800 μL 0.9% saline → vortexed → then 200 μL of this suspension is pipetted into a fresh tube containing 1800 μL 0.9% saline, etc.) until a final inoculum of 1.5 x102 CFU/ml is reached.
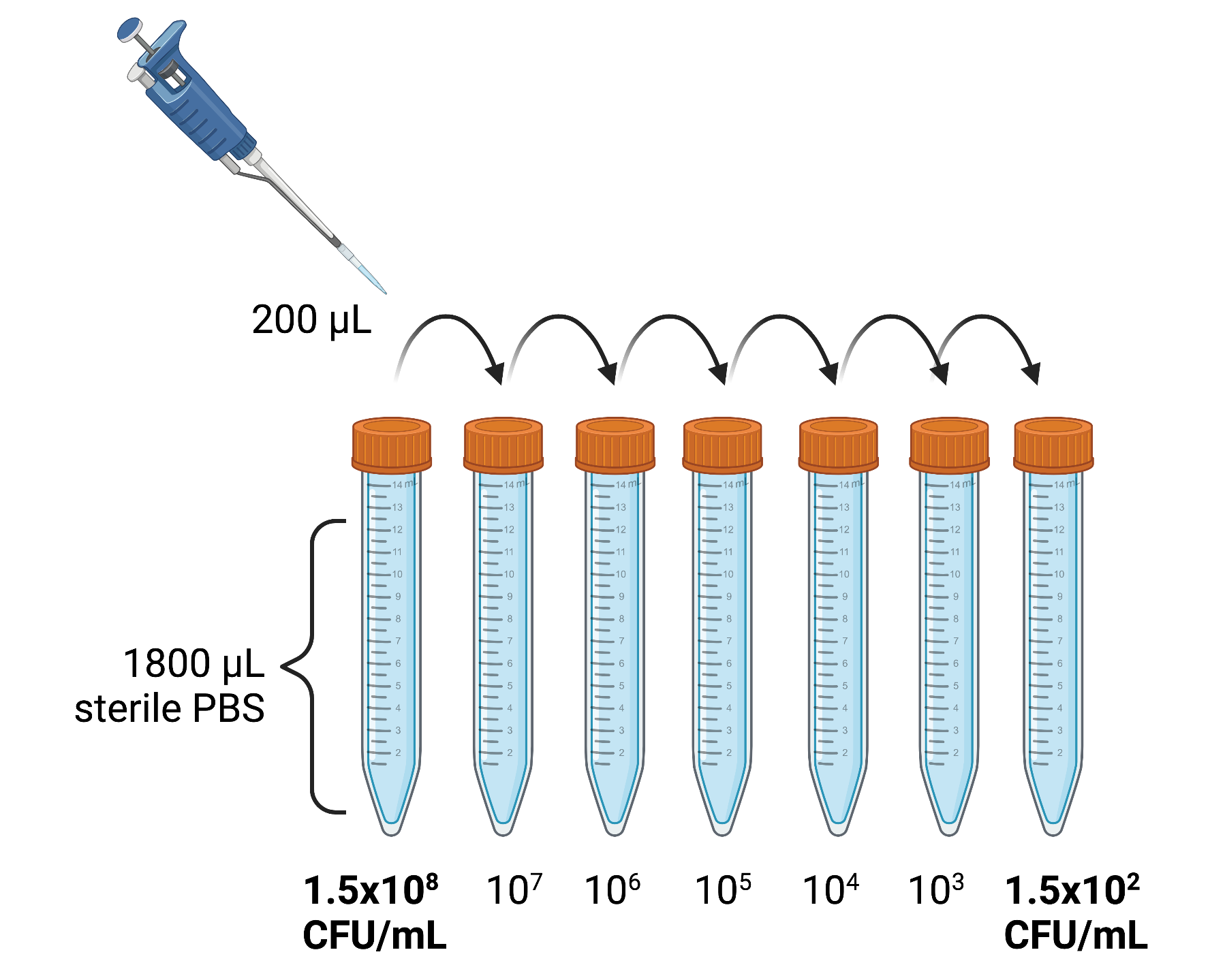
Note the final dilution step is repeated an additional 5 times in replicate tubes to ensure 6 tubes (12 mL of the final 1.5 x102 CFU/mL) are available to inoculate 10 blood culture bottles.
Antibiotic preparation
Analytical grade (> 99% pure) ceftazidime (200 μg/mL) and avibactam (500 μg/mL) pure powder are diluted separately in sterile water to prepare a stock solution of ceftazidime (200 μg/mL) and avibactam (500 μg/mL). The stock solution is dispensed into 2 mL sterile cryo-vials and stored at -20°C.
Frozen stock antibiotic solution in cryo-vials is thawed the day of the experiment and diluted at twice the final test concentration using either (a) pooled patient serum; or (b) 0.9% PBS.
Example: to obtain 2 mL of 20 mcg/ml stock: 200 μL of ceftazidime 200 mg/mL solution is pipetted into a test-tube containing 1800 μL of serum
Example to obtain 50 μg/mL: using the multipipettor set at 500 μL, 500 μL of ceftazidime 200 μg/ml solution are added in a tube containing 1500 μL of serum
500 μL of the ceftazidime 2x concentration and 500 μL of the 2x avibactam concentration are then added in a test tube to obtain 1 ml of serum-antibiotic solution at the desired concentrations
After vortexing for 7-10 seconds each tube, 1 ml of inoculum suspension at 1.5x102 CFU/mL and 1 mL of antibiotic containing serum are inoculated with a sterile insulin syringe into a blood culture bottle containing 40 mL of enrichment broth without inactivating matrix.
Bloodculture bottles are then incubated into Bact/ALERT system to detect growth. The same procedure is repeated for all the selected antibiotic concentration-inoculum pairs.
One bottle is inoculated with the bacterial inoculum without adding the antibiotic solution (drug-free control).
Inoculum preparation workpackage 1A.
To obtain a series of ten-fold diluted inoculum to be inoculated in each blood culture bottle.
Base inoculum: Colonies are selected from an 18- to 24-hour agar plate and suspended in saline solution 0.9% in a glass test tube. The suspension is adjusted by optimal density by the Densichek plus instrument to achieve turbidity equivalent to a 1 McFarland standard that corresponds to 3 × 108 CFU/mL.
The inoculum is then serially ten-fold diluted in 0.9% saline to obtain subsequent inocula down to 3 x101 CFU/ml.
Using a multipipettor, 200 μL are withdrawn from tube 1 (inoculum 3 x108 CFU/mL) and added to tube 2 which contains 1800 μL of saline solution (3x107 CFU/mL). The same procedure is repeated serially to produce an inoculum dilution series down to 3 x101 CFU/mL. With each pipette transfer, the suspension is mixed up and down 6-8 times before the subsequent transfer of 200 μL.
1 ml of each dilution is inoculated with a sterile 1 ml syringe into a blood culture bottle for aerobic incubation (BacT/ALERT FA FAN® Aerobic Biomeriuex)
Blood culture bottles are incubated into the Bact/ALERT 3D Biomerieux incubator system for 24 hours that automatically reports time to positivity;
The day after, time to positivity (defined as the time from the start of incubation to detection of growth) is reported for every bottle.
Inoculum preparation workpackage 1A.
Inoculum preparation: to obtain 1ml of inoculum of 1.5 x104 CFU/mL to be inoculated in each blood culture bottle. The procedure is partly similar to methodology for Workpackage 1A.
Base inoculum: Colonies are selected from an 18- to 24-hour agar plate and suspended in saline solution 0.9% in a glass test tube. The suspension is adjusted by optimal density by the Densichek plus instrument to achieve turbidity equivalent to a 0.5 McFarland standard that corresponds to 1 to 2 × 108 CFU/mL.
The inoculum is then serially ten-fold diluted 0.9% saline to obtain a final inoculum of 1.5 x104 CFU/ml.
Using a multipipettor, 300 μL are withdrawn from tube 1 (inoculum 1.5 x108 CFU/mL) and added to tube 2 which contains 2700 μL of saline solution (1.5x107 CFU/mL). The same procedure is repeated serially to produce an inoculum dilution series down to 1.5 x105 CFU/mL. With each pipette transfer, the suspension is mixed up and down 6-8 times before the subsequent transfer or 300 μL. Last dilution is performed by withdrawing 300 μL from tube 4 and adding this to tube 5 which contains 2700 μL of MH broth; since the glass tubes contain a maximum of 3-4 mL, this last step is repeated for at least 4 tubes in parallel to obtain a total amount of 12 mL of inoculum 1.5 x104 CFU/ml (approximately 10 antibiotic dilutions and 10 blood culture bottles that are inoculated with 1 mL of bacterial inoculum).
Alternative procedure: last dilution is performed by withdrawing 1 mL from tube 4 and adding this to a 15 mL sterile falcon tube that contains 9 mL of MH broth; this last step is repeated twice and it permits to obtain a total amount of 20 mL of inoculum 1.5 x104 CFU/ml.
Antibiotic preparation
Antibiotic dilutions
- ceftazidime and avibactam pure powder are diluted in sterile water and conserved at -20°C
- Stocked 200 mcg/ml of ceftazidime solution and 500 mcg/ml of avibactam solution are thawed at room temperature
- Further dilution to obtain the desired concentrations: antimicrobial solutions are calculated at twice the desired final concentration
For example: from ceftazidime 200 μg/mL, how to obtain 2 mL of 20 μg/mL stock: using the multipipettor set at 200 mcl, 200 mcl of ceftazidime 200 mcg/mL solution are added in a tube containing 1800 μL of serum.
For example: from ceftazidime 200 μg/ml, how to obtain 50 mcg/ml: using the multipipettor set at 500 μL, 500 μL of ceftazidime 200 μg/mL solution are added in a tube containing 1500 μL of serum.
- 500 μL of the doubled desired ceftazidime concentration and 500 μL of the doubled desired avibactam concentration are added in a test tube to obtain 1 ml of serum-antibiotic solution at the desired concentrations
Alternative procedure: ceftazidime and avibactam pure powders are diluted in sterile water and conserved at -20°C
Stocked ceftazidime and avibactam solutions are thawed at room temperature
Considering the blood culture bottles containing 40 mL of enrichment broth, antibiotic stocked solutions are further diluted to obtain concentrations 40 times the MIC values or its multiples.
Example: ceftazidime pure powder is diluted to obtain a concentration of 320 μg/mL in 1 mL of serum that corresponds to a final concentration of 8 μg/ml after inoculation into the blood culture bottle.
- Antimicrobial solutions are calculated at twice the desired final concentration; 500 μL of the doubled desired ceftazidime concentration and 500 μL of the doubled desired avibactam concentration are added in a test tube to obtain 1 mL of serum-antibiotic solution at the desired concentrations
Example: to test a final concentration of ceftazidime 2 μg/mL and avibactam 0.5 μg/mL, ceftazidime and avibactam are respectively diluted to obtain a concentration of 160 μg/mL and 40 μg/mL. 500 μL of each antibiotic are added in a test tube to obtain a concentration of 80-20 μg/mL which correspond to a final concentration inside the blood culture bottle of 2-0.5 μg/mL.
- After vortexing for 7-10 seconds each tube, 1 ml of inoculum suspension at 1.5x104 CFU/ml and 1 ml of serum with antibiotic concentration are inoculated with a sterile 1 ml insulin syringe into a blood culture bottle containing 40 ml of enrichment broth without inactivating matrix (BacT/ALERT SA Standard Aerobic Biomerieux) and incubated into the Bact/ALERT 3D Biomerieux incubator system for 24 hours that automatically detects growth.
- The same procedure is repeated for all the selected antibiotic concentration-inoculum pairs; one bottle is inoculated with the bacterial inoculum adding 1 mL of saline solution without antibiotic as control.
- The day after, time to positivity (defined as the time from the start of incubation to detection of growth) is reported for every bottle; bottles showing no growth at 24 hours are reported as “24”.